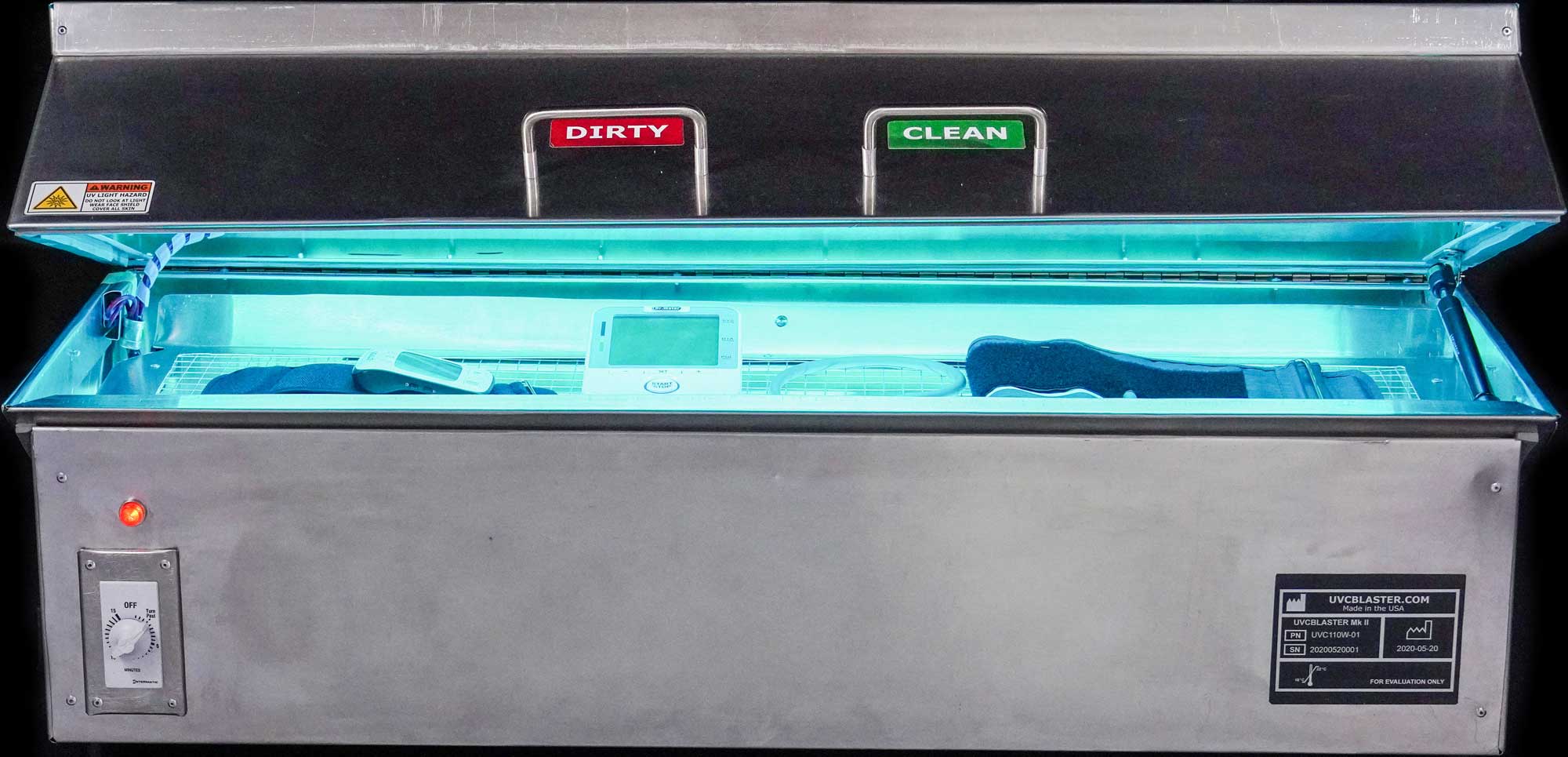
If the device includes a UV lamp, FDA recommends that the manufacturer evaluate whether the product controls for time, UV radiation dose, and intensity of UV dose, and validates the cleaning and disinfection procedures.
a) A caution that UV disinfection will reduce the number of pathogens on the device, but it will not eliminate them completely.
b) A statement that the device is an adjunct to currently existing reprocessing practices and not a replacement or modification to such practices.
c) A statement regarding the time, distance, and maximum area over which the device has been evaluated for effectiveness.
d) An appropriate UV hazard warning label.
e) Identification of the expected UV lamp operational life and instructions for procedures on replacement of the UV lamp when needed.
f) Procedures to follow if the UV lamp malfunctions or fails.
g) Description of the preparation of equipment or the room for disinfection
h) A statement that the equipment intended to be disinfected is UV compatible.
i) Identification of the UV dose.
UV disinfecting devices include UV radiation chamber disinfection devices, which are regulated as Class II devices under 21 CFR 880.6600 (product code OSZ).